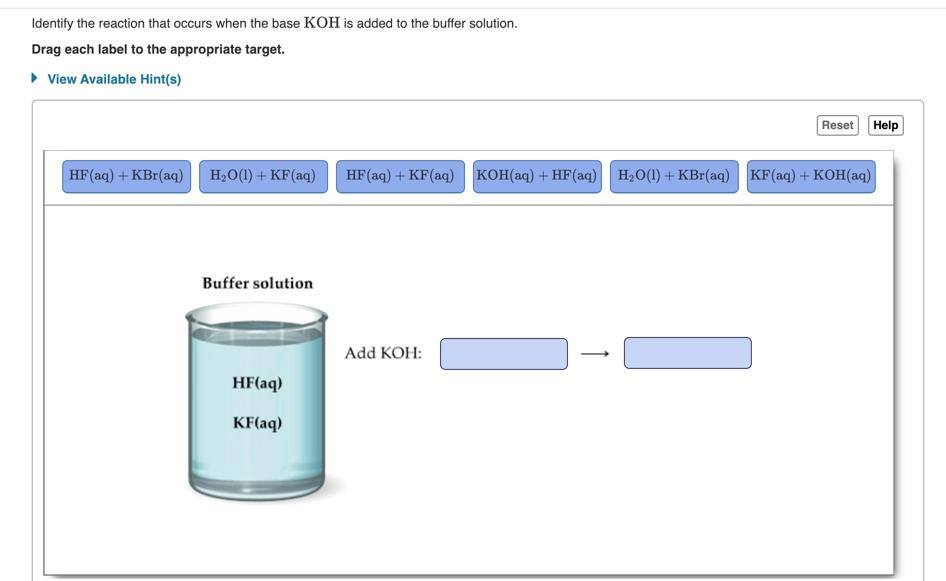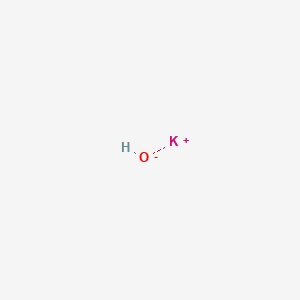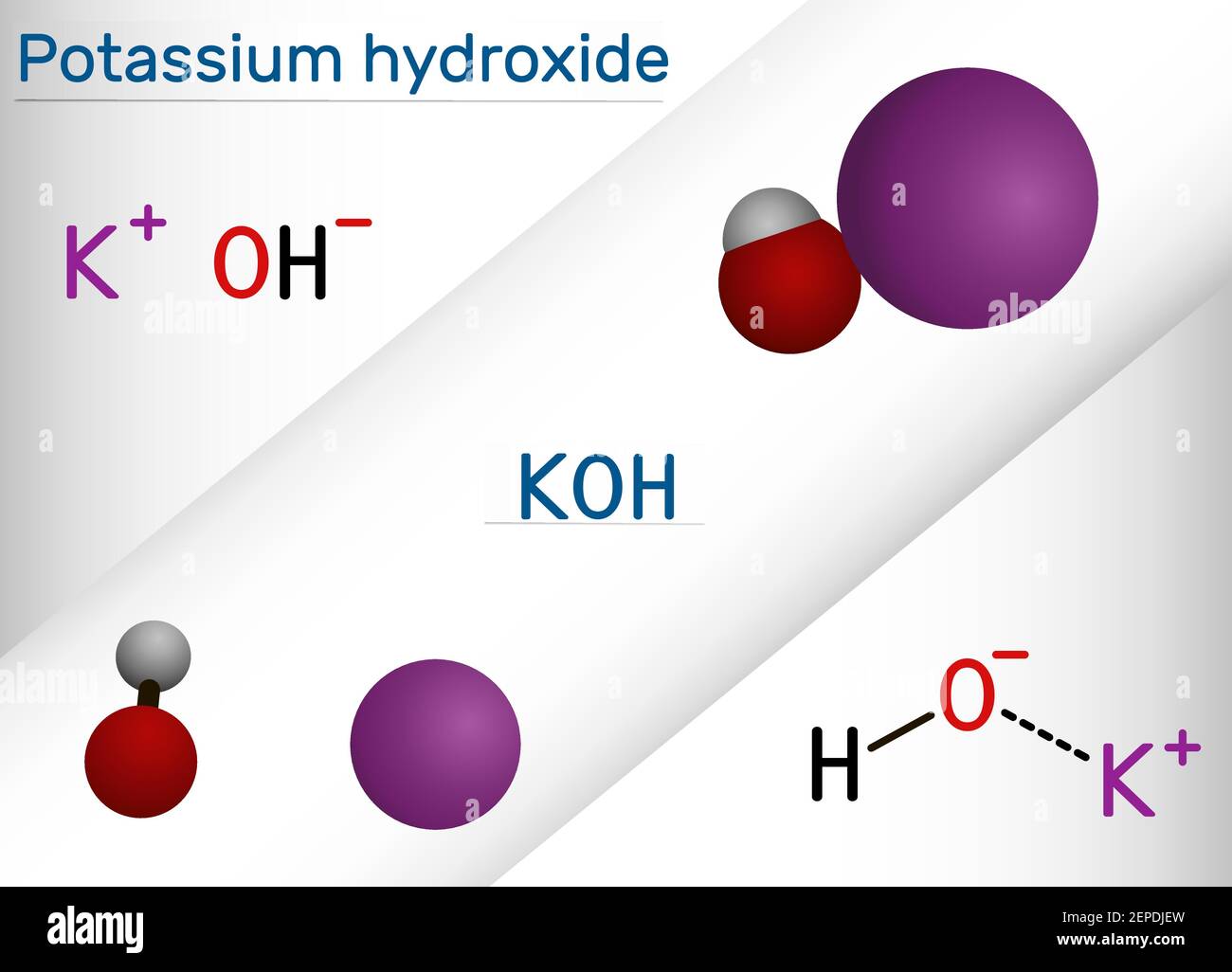
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
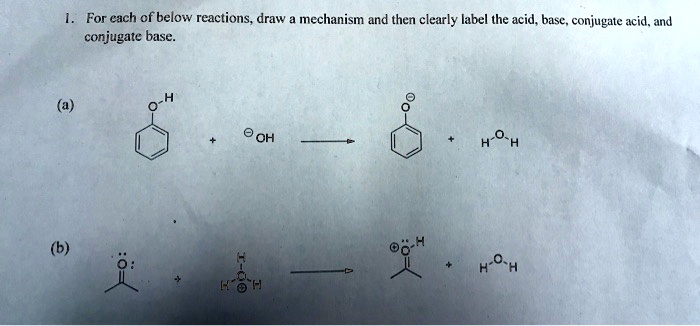
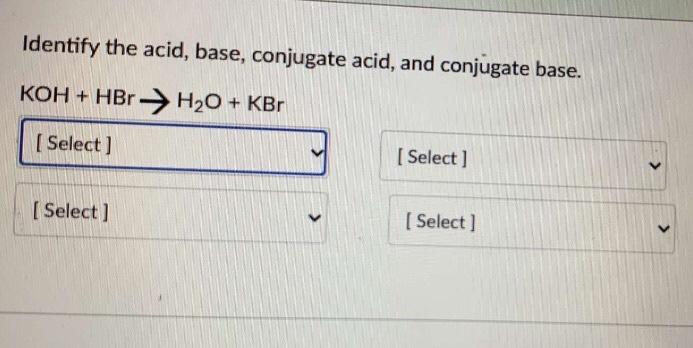
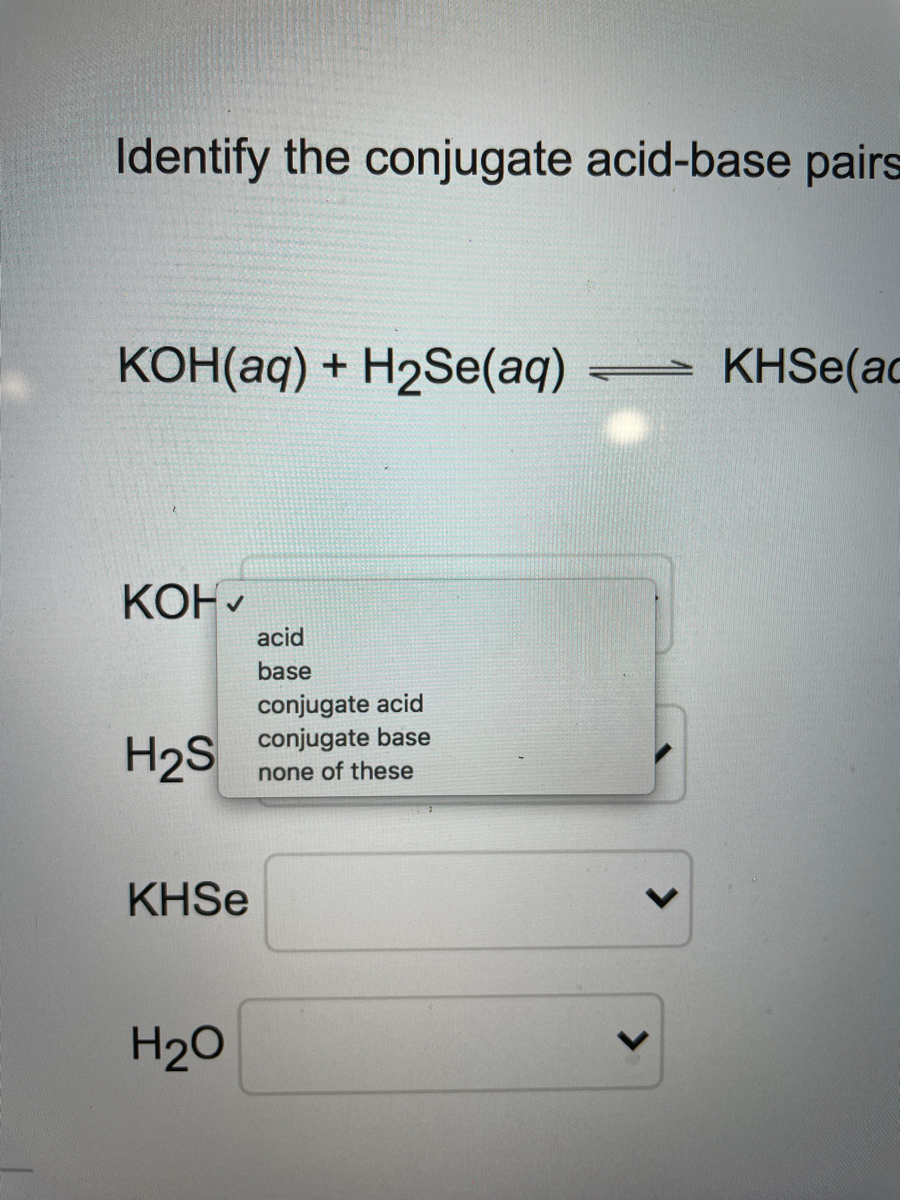
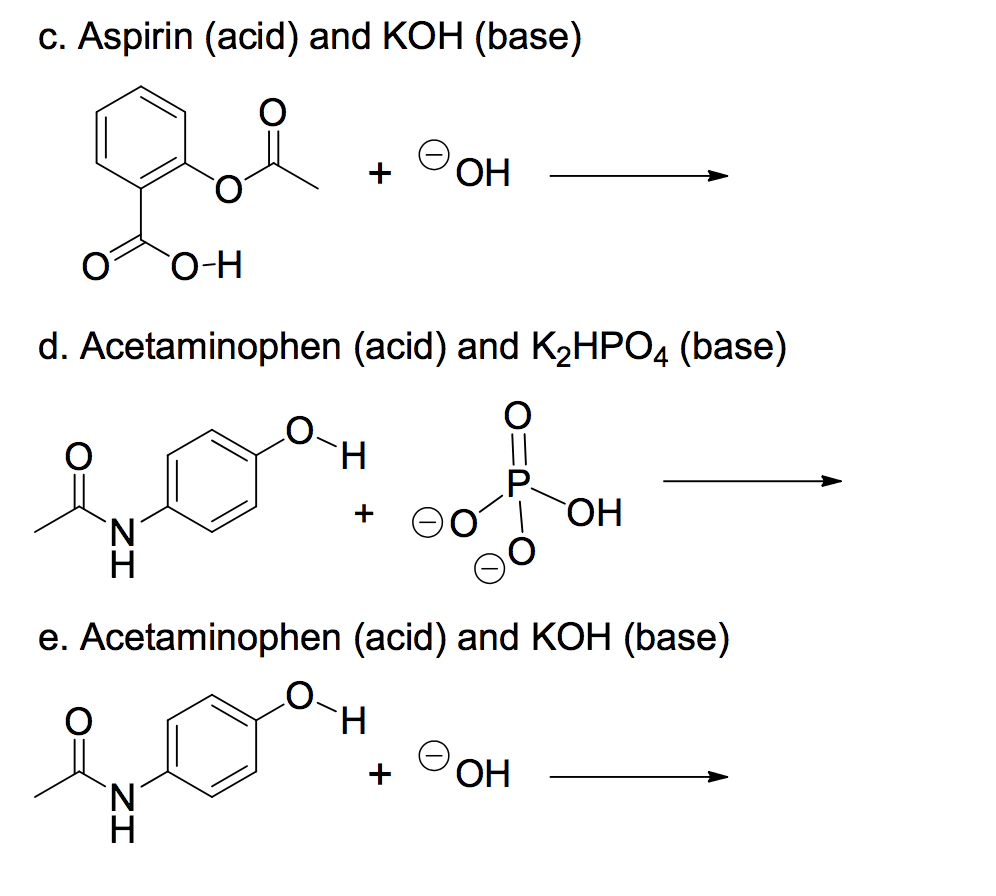
SOLVED: For each of below reactions, draw mechanism and then clearly label the acid; base, conjugate acid, and conjugate base. OH Koh

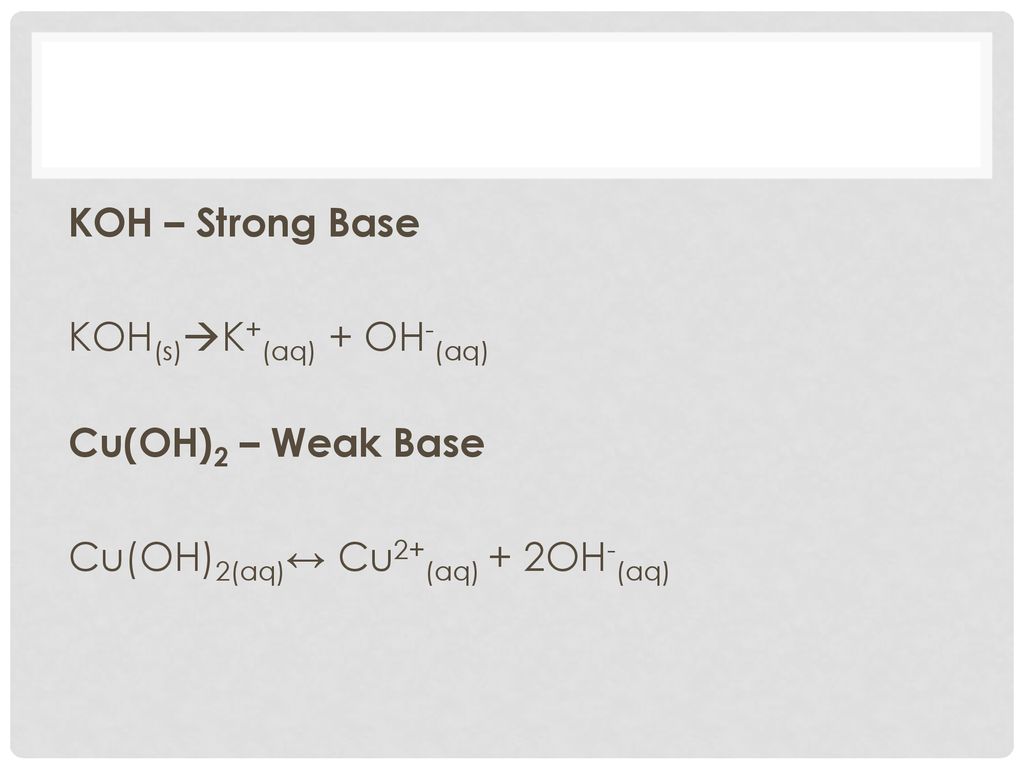
SOLVED: Potassium hydroxide dissociates in water to produce hydroxide ions. KOH(s) + H2O(l) â†' K+(aq) + OH-(aq) Given the information above, how is potassium hydroxide categorized? A. Both a Bronsted-Lowry base and

Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com

SOLVED: Potassium hydroxide dissociates in water to produce hydroxide ions. KOH(s) + H2O(l) â†' K+(aq) + OH-(aq) Given the information above, how is potassium hydroxide categorized? A. Both a Bronsted-Lowry base and
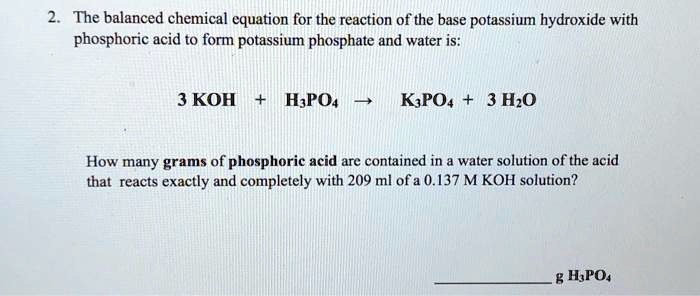
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH + H3PO4 -> K3PO4 + 3 H2O
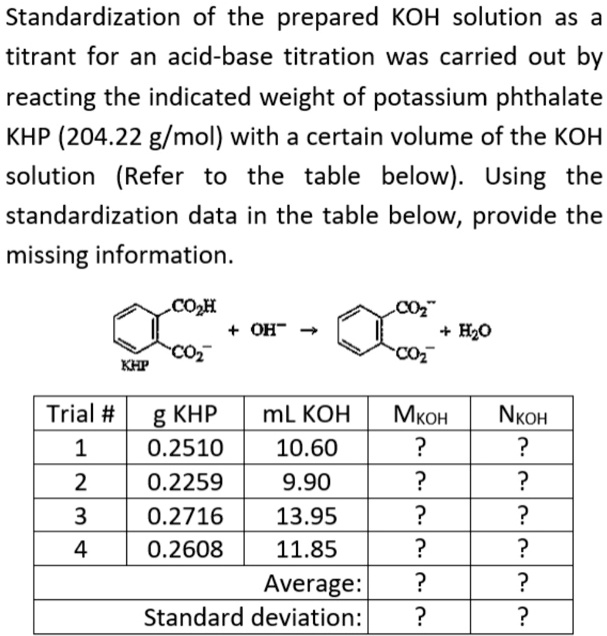
SOLVED: Standardization of the prepared KOH solution as a titrant for an acid-base titration was carried out by reacting the indicated weight of potassium phthalate KHP (204.22 g/mol) with a certain volume
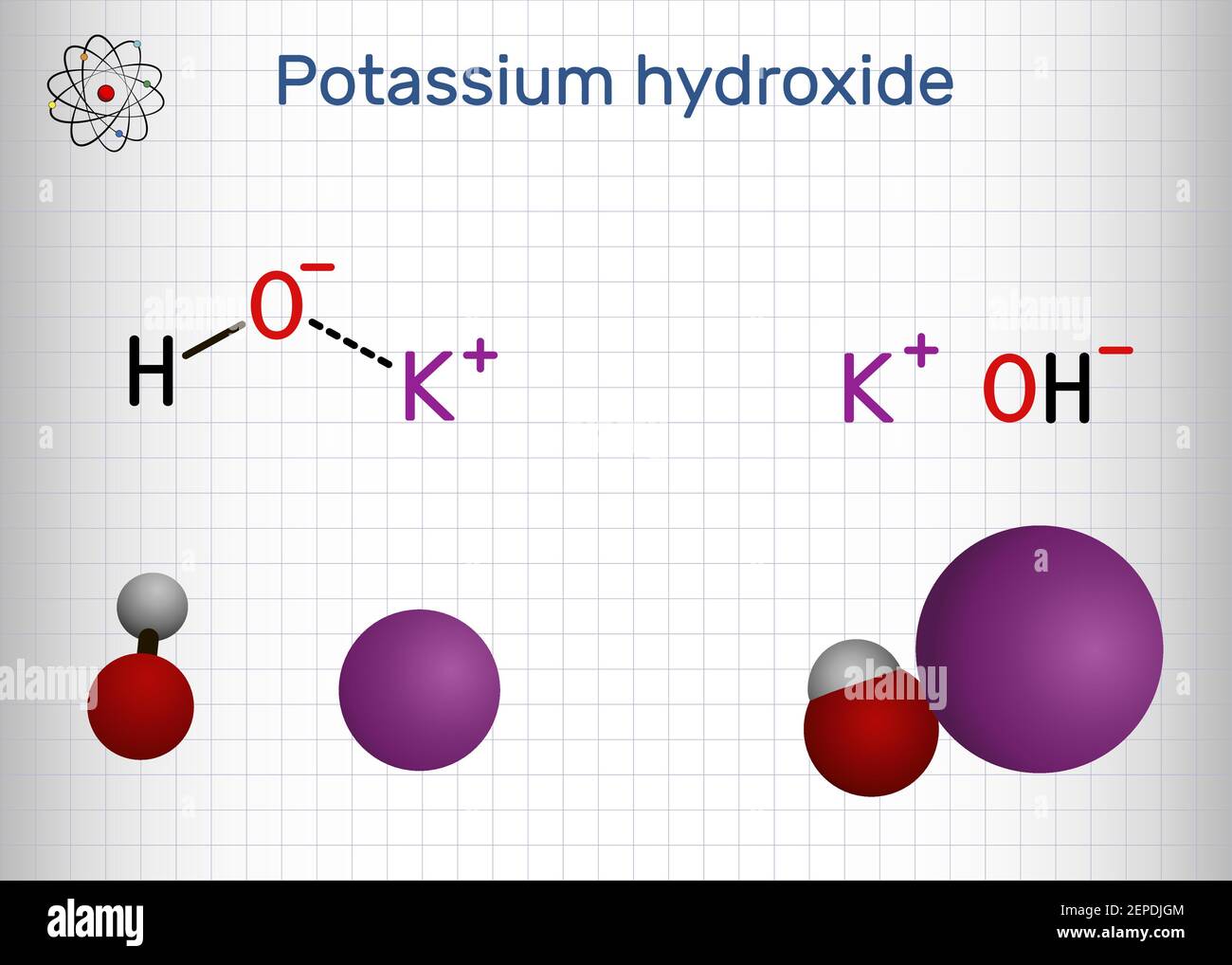
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy